Br CI I in methanol. Likewise if youre dealing with a reaction between a neutral molecule and a positive ion cation then the neutral molecule will have a generally higher electron density and will act as a nucleophile.
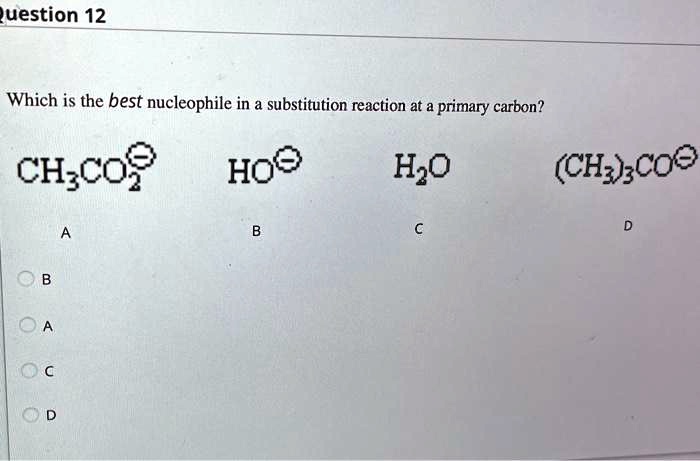
Solved Uestion 12 Which Is The Best Nucleophile In A Substitution Reaction At Primary Carbon Ch Co Hoo Hzo Chz Scoe
The strongest nucleophile among the following is.

. The strongest nucleophile among the following is. Oxygen would rather KEEP its electrons. The halogens iodine and bromine are both very good leaving groups.
Smaller molecules are better nucleophiles than larger ones they are not as sterically hindered. CH3CO CHCHS CHCHO in methanol c. Which of the following is the best nucleophile in an SN2 reactionA.
All the four species are nucleophiles as they contain either lone pairs or lone pairs and negative charge which can be easily donated to the suitable electrolyte. Rank the following species in each set from best nucleophile to poorest nucleophile. The best leaving group is the atomion that will be the most stable species when it is kicked out of the substrate.
Among the following the strongest nucleophilic is. This means that electrons will not be tightly held together and will rather donate them. When comparing the nucleophilicity of two molecules where the atoms donating the electrons are in the same row of the periodic table the less electronegative atom holds onto its electrons less tightly and therefore is a better nucleophile.
Wants to give away electrons good Lewis base. Rank the following species in each set from best nucleophile to poorest nucleophile. 1 Chemical Foundations 2 Atoms Molecules And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding.
The ability for an atom slash ion slash molecule to act as a nucleophile or to give away extra electrons and bond with a nucleus or with something else. This is relative because nucleophilic strength is also dependent on other factors in the reaction such as solvent. Bigger So I-is a better nucleophile than F-.
Some examples of compounds wherein carbon acts as a nucleophile include Grignard Reagents Organolithium Reagents and n-butyllithium. Therefore fluoride is the best nucleophile and iodide the best leaving group. C Ph3 Scontains a negative charge and lob pair of electrons.
Due to which the solvation effect become less. Iodine with negative charge having more nucleophilicity because it has a bigger size. Thus the lone pair of.
HO- better than H2O bases always better than their conjugate acids better than less electronegative atom means more willing to give up e- HS-better than Cl-NH3 H2O What Makes a Good Nucleophile. Up to 10 cash back The ordering from best nucleophile to worst nucleophile is as follows. Oxygen The hydroxide ion is a great example of a nucleophile wherein the electron pair is donated by the oxygen atom.
For the rest three we are looking at the pna values of their conjugate acid. It is the best nucleophile because it has a negative charge more electron density and its electrons are held less tightly than those of CH 3 O because sulphur is less electronegative than oxygen. Which of the following alkyl halides would be expected to react the fastest under SN2 conditions.
Nucleophiles all have pairs of electrons to donate and tend to be. It is a nucleophile reactant that provides a couple of electrons to form a new covalent bond we can say that it acts as a Lewis base. View the full answer.
Is a better nucleophile than because nitrogen is less electronegative than oxygen Look for the the lower electronegativity on the atom holding the lone pair of electrons. A carboxylic is a stronger acid than phenoe which is stronger acid than water. Chlorine is a good leaving group and fluorine is a poor leaving group.
CH 3 S. So in this reaction the negative oxygen is our nucleophilic piece while the carbon attached to bromine is going to be an electrophile. The better nucleophile would be CH3SH because S is less electronegative than oxygen.
NH 3 CH 3 S CH 3 SH H 2 O CH 3 O. The poorest nucleophile is the one with the neutral oxygen. RCO 2 is a better nucleophile than RCO 2 H.
A Lewis base contains at least one lone pair of electrons and a Lewis acid is a species that contains an atom that is at least two electrons short of a closed outer shell. A Phosphine contains lone pair of electrons on P atom B Fcontains a negative charge and three lone pairs of electrons. 2nucleophile means searching of nucleus so here only OH - having negative charge.
A good base is usually a good nucleophile. RO OH RLi RCC and NH₂. Below is a table of relative nucleophilic strength.
General Concepts 9. Because water is the weakest acid its conjugate base is the strongest base and the best nucleophile. As shown above as a general rule the anion of a reactant will be a better nucleophile than the neutral form.
So strong bases substances with negatively charged O N and C atoms are strong nucleophiles. Which of the following compounds would be the best nucleophile. And - in DMSO d.
What Makes a Good Nucleophile. I want to say with a nucleus because thats what nucleophilicity is saying. H2O and NH3 in methanol b.
Carbon carbon acts as a nucleophile in many organometallic reagents and also in enols. Also the question never told us which solvent the nucleophile was in. Or Ill just make up a definition right now.
Ill say with a nucleus. Among the following the strongest nucleophilic is. Chemistry questions and answers.
Some strong bases are poor nucleophiles because of steric hindrance.

What Makes A Good Nucleophile Master Organic Chemistry Organic Chemistry Chemistry Organic Chemistry Books
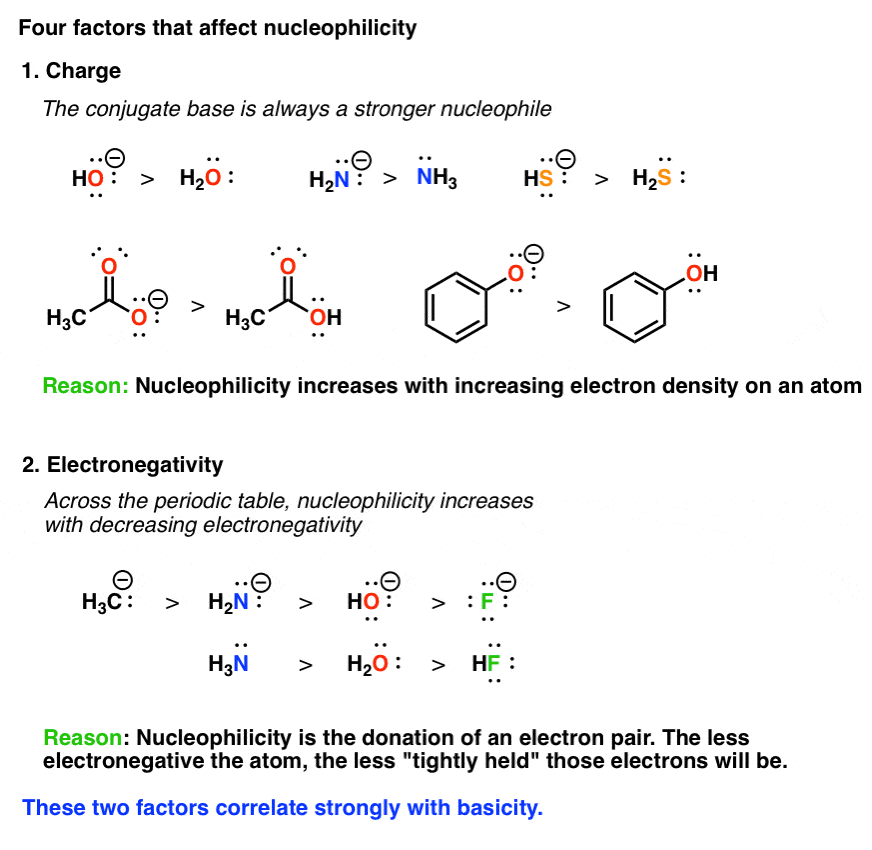
What Makes A Good Nucleophile Master Organic Chemistry

Image Result For Nucleophiles Organic Chemistry Chemistry Hydrogen Bond
0 Comments